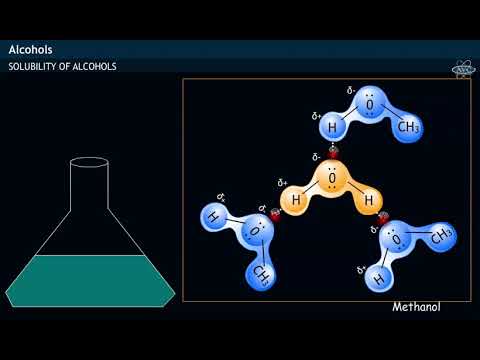
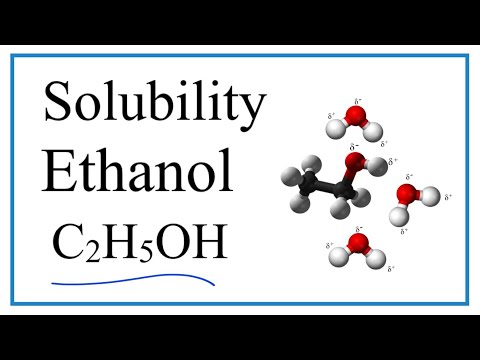
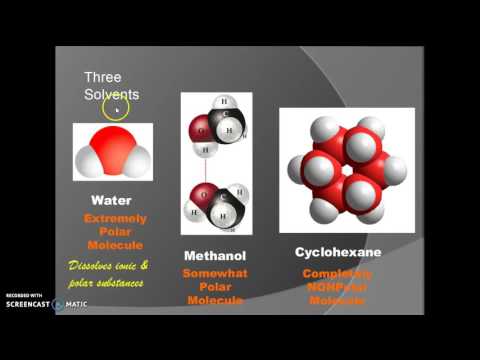
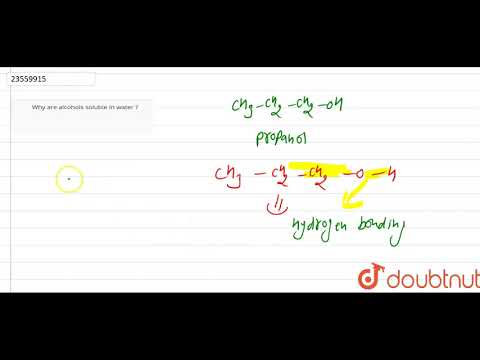
関連ワード:
is methyl alcohol soluble in water how is amyl alcohol soluble in water is methyl alcohol miscible in water does methyl alcohol dissolve in water is n amyl alcohol soluble in water is t amyl alcohol soluble in water is meoh soluble in water is methanol ch3oh soluble in water is methanol soluble in water is ch3oh soluble in water