関連ワード:
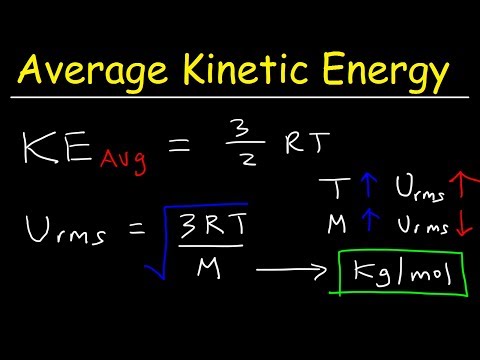
what is rms speed of gas molecules what is root mean square speed of gas molecules what is the rms speed of helium gas molecules at stp rms speed of gas molecules formula rms speed of gas molecules class 11 rms speed of gas molecules derivation rms speed of gas molecules class 11 physics rms speed of gas molecules calculator rms speed of gas molecules class 11 derivation at what temperature is the rms speed of gas molecules half the value at ntp