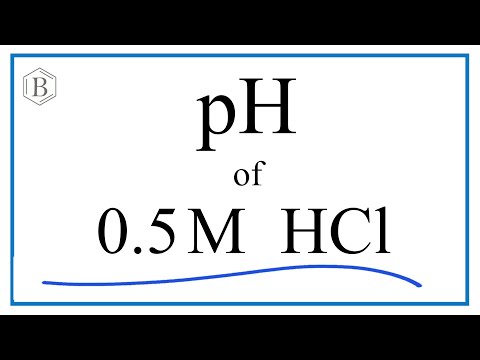
結果 : what is the ph of a 0.3 m hydrochloric acid solution

1:11
Find the pH of a 0.03M HCl (Hydrochloric acid) Solution
Wayne Breslyn (Dr. B.)
2,426 回視聴 - 1 年前

1:37
Find the pH of a 0.1M HCl (Hydrochloric acid) Solution
Wayne Breslyn (Dr. B.)
16,214 回視聴 - 1 年前

1:05
Find the pH of a 0.05M HCl (Hydrochloric acid) Solution
Wayne Breslyn (Dr. B.)
4,836 回視聴 - 1 年前