関連ワード:
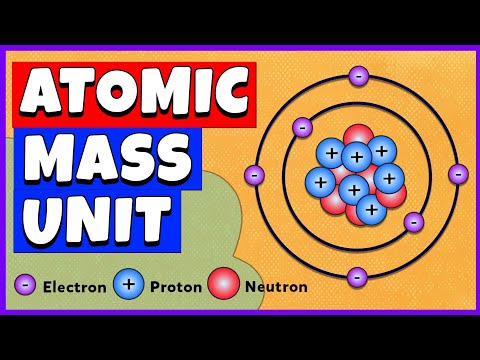
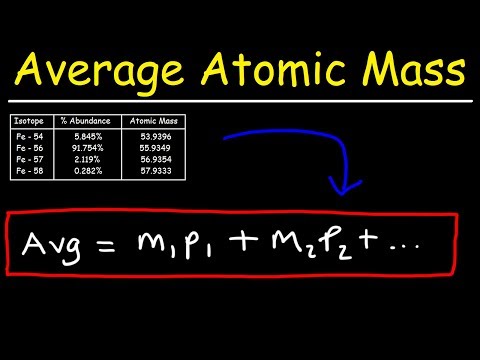
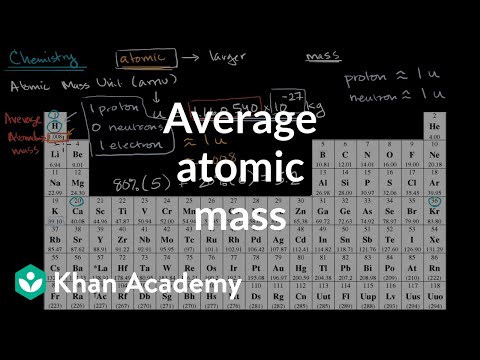
why is the atomic mass unit defined by c12 isotope why is the atomic mass unit defined by c12 isotope class 9 why is the atomic mass unit defined by carbon 12 isotope why is the atomic mass unit defined by carbon 12 isotope class 9 why is the atomic mass unit defined by carbon c12 isotope why is carbon chosen for atomic mass unit why atomic mass unit is needed