関連ワード:
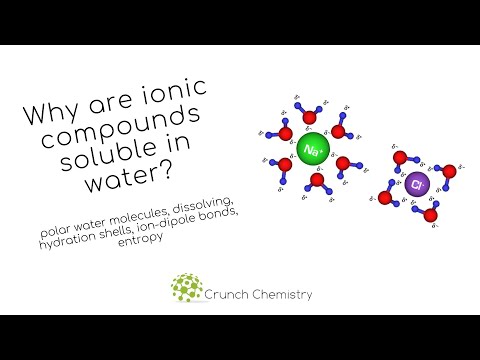
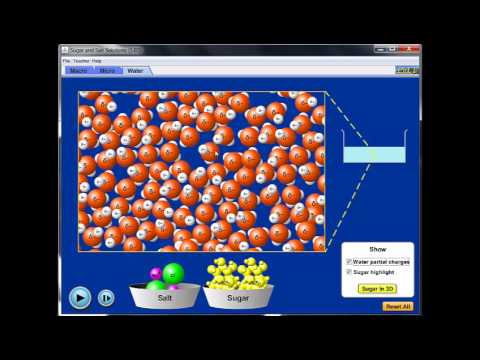
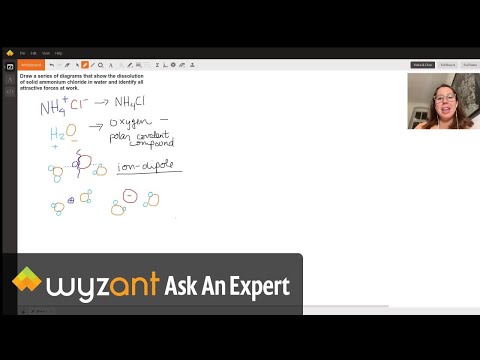
are ionic compounds more soluble in water than covalent are ionic or covalent compounds more soluble in water ionic bonds are more soluble in water than covalent bonds compounds containing covalent bonds are more soluble in water than those with ionic bonds why are ionic compounds more soluble in water than covalent compounds why are ionic compounds more soluble in water are ionic bonds soluble in water