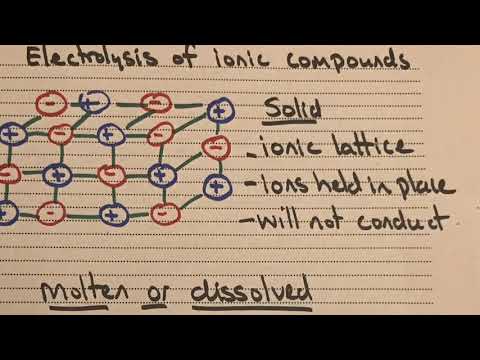
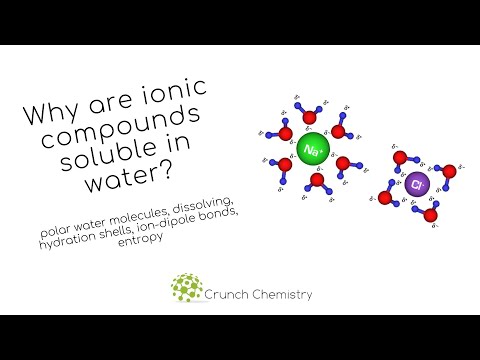
関連ワード:
do ionic compounds conduct electricity when dissolved in water do ionic bonds conduct electricity when dissolved in water can ionic compounds conduct electricity when dissolved in water why do ionic compounds conduct electricity when dissolved in water but not as a solid do all ionic compounds conduct electricity when dissolved in water why do ionic compounds conduct electricity when dissolved in water or melted do ionic compounds only conduct electricity when dissolved in water do ionic compounds always conduct electricity when dissolved in water why do ionic compounds conduct electricity when dissolved in water or molten do ionic or covalent compounds conduct electricity when dissolved in water