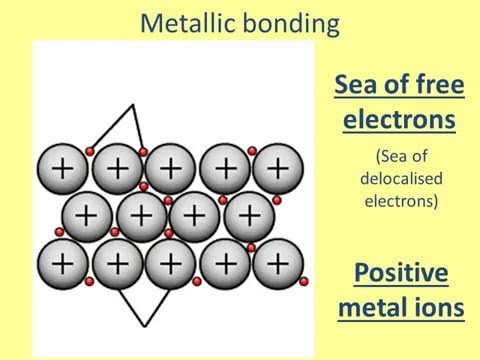
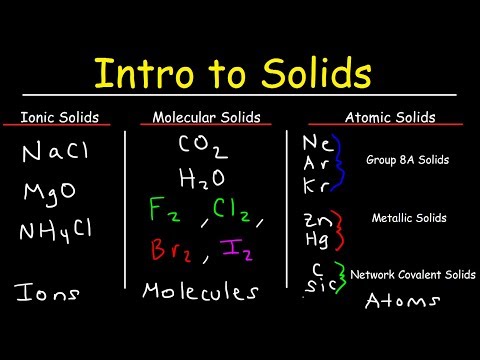
関連ワード:
does ionic bond conduct electricity in solid state does ionic compound conduct electricity in solid state do ionic compounds conduct electricity in solid state do ionic compounds conduct electricity in solid form ionic bond conduct electricity in solid state do ionic compounds conduct electricity in solid or molten state why do ionic bonds not conduct electricity in solid state why does ionic compound not conduct electricity in solid state why do ionic compounds not conduct electricity in solid state why do ionic compounds not conduct electricity in solid state class 10