関連ワード:
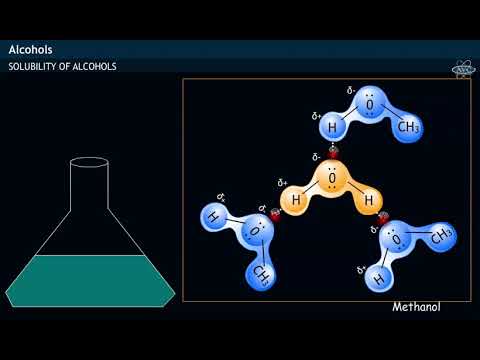
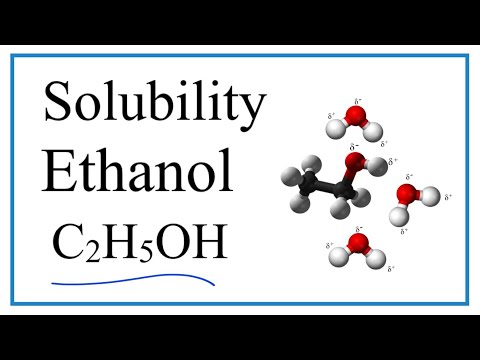
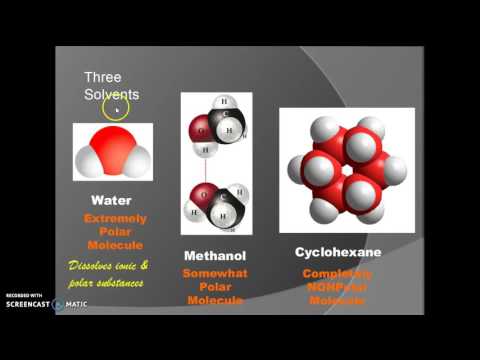
does methanol dissolve in water does methyl alcohol dissolve in water can methanol dissolve in water and conduct electricity does methanol remove water why does methanol dissolve well in water why does methanol not dissolve in water what does methanol dissolve into in water does methanol conduct electricity when dissolved in water methanol dissolve in water methanol dissolve in water equation