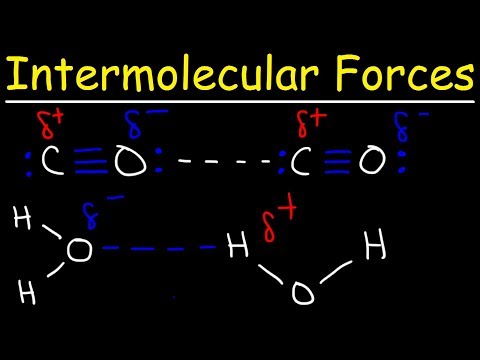
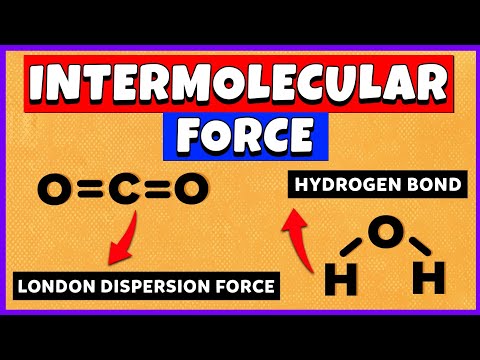
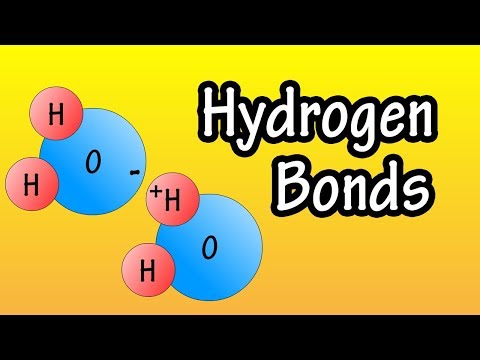
関連ワード:
does water or alcohol have stronger intermolecular forces does water or ethanol have stronger intermolecular forces does water or isopropyl alcohol have stronger intermolecular forces does water or ethyl alcohol have stronger intermolecular forces does water or rubbing alcohol have stronger intermolecular forces does water have stronger intermolecular forces than alcohol does water have stronger intermolecular forces than isopropyl alcohol does water have stronger intermolecular forces than ethanol which liquid water or alcohol has stronger intermolecular forces which liquid water or alcohol has stronger intermolecular forces explain