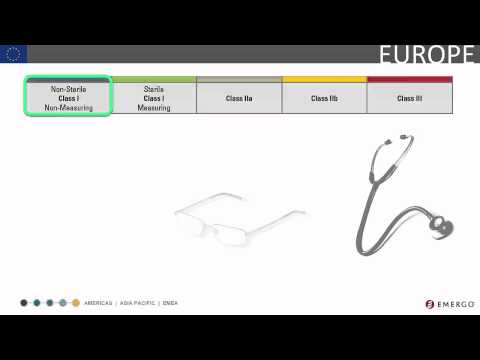
結果 : eu mdr classifications

34:22
How to make labelling regulatory compliant for EU-MDR (2017/745)
Decos Global
1,654 回視聴 - 1 年前

44:58
Unleashing "MDR classification surprises" with Frank Matzek
Easy Medical Device
743 回視聴 - 2 年前

36:29
Clinical Evaluation of Medical Devices according to EU MDR 2017 745
Asphalion
1,635 回視聴 - 4 年前