関連ワード:
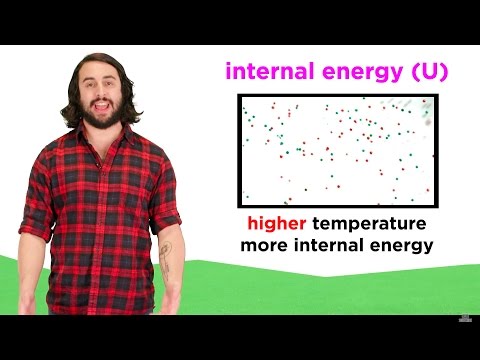
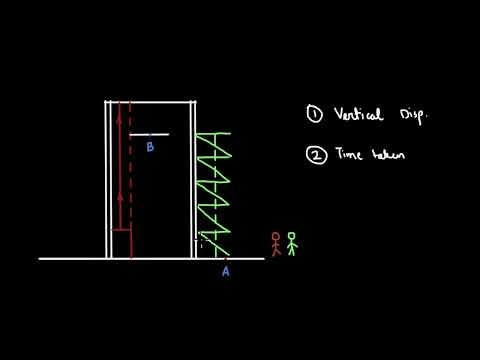
explain why heat and work is not a state function explain why energy is a state function but heat and work are not explain why energy is a state function and why heat and work are not why heat and work are not state functions why is work not a state function why is heat not a state function explain why heat capacity is not a state function