関連ワード:
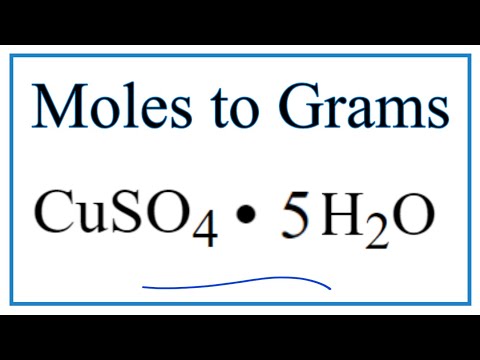
gram molecular weight of cuso4 5h2o gram molecular mass of cuso4 5h2o calculate molecular mass of cuso4 5h2o calculate molar mass of cuso4 5h2o calculate the molecular weight of cuso4 5h2o calculate the gram molecular mass of cuso4 5h2o calculate relative molecular mass of cuso4 5h2o weight of cuso4.5h2o