関連ワード:
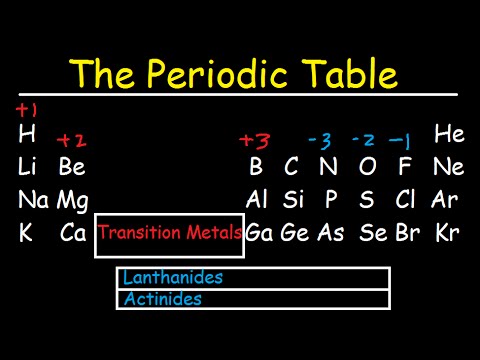
how are elements in the same group similar how are elements in the same group similar to each other how are elements in the same period similar how are elements in the same group of the periodic table similar how do elements in the same group have similar properties how do elements in the same group react similarly how are elements in groups similar are elements in the same group have similar properties are elements in the same group chemically similar are elements in the same group have similar chemical properties