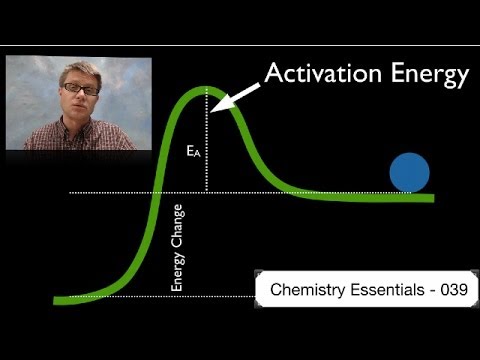
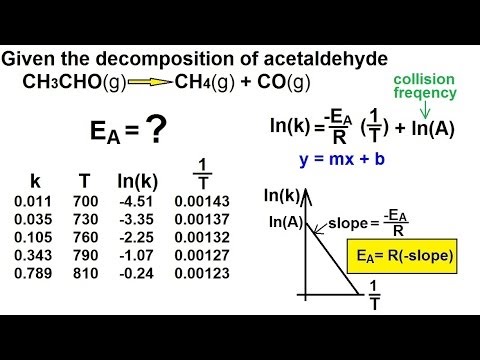
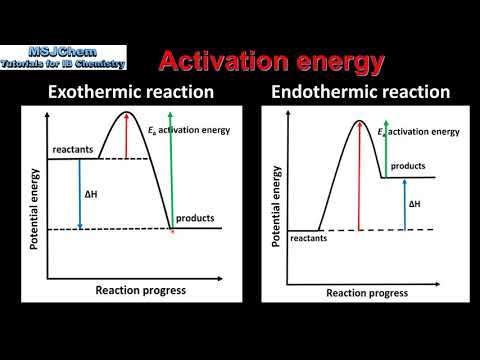
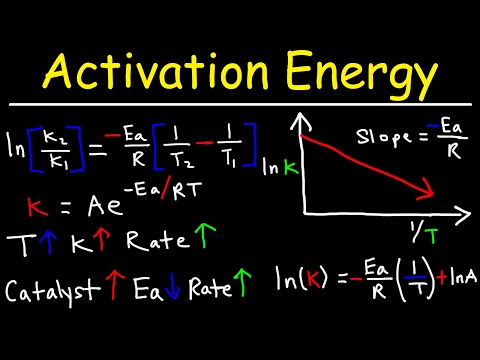
関連ワード:
how to calculate activation energy in chemistry how to find activation energy in chemistry how to calculate activation energy higher chemistry how to calculate activation energy ib chemistry how to calculate activation energy a level chemistry how to calculate activation energy from a graph chemistry how to find activation energy from a graph chemistry how do i calculate activation energy what is activation energy in chemistry formula for calculating activation energy