関連ワード:
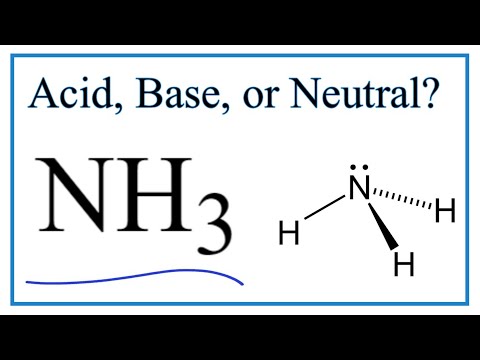
is ammonia a weak base in water is nh3 a strong base in water is nh3 a weak base in aqueous solution is ammonia a weaker base than water ammonia is classified as a weak base in water this means that it produces is sodium hydroxide a weak base and is water soluble ammonia is a weak base that reacts with water sodium hydroxide is classified as a strong base in water this means that it produces what is a characteristic of a weak base like ammonia in water is ammonia a weak base