関連ワード:
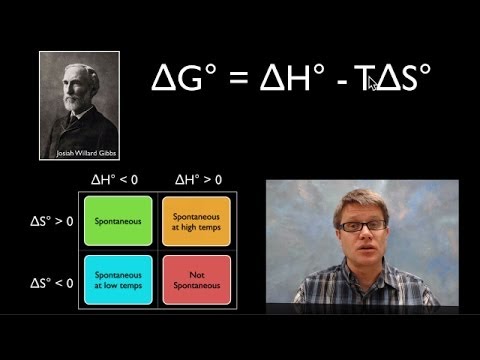
is delta g spontaneous when negative is delta g spontaneous when negative or positive is delta g spontaneous if negative is negative delta g spontaneous or nonspontaneous is gibbs free energy spontaneous when negative if delta g is negative is the reaction spontaneous if delta s is negative is the reaction spontaneous