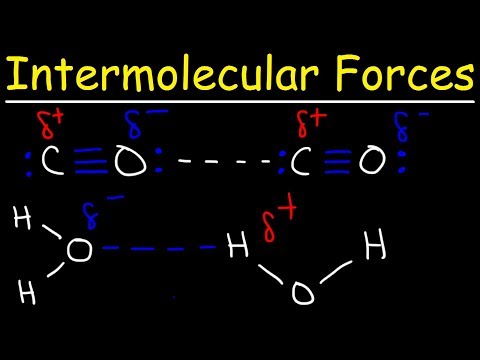
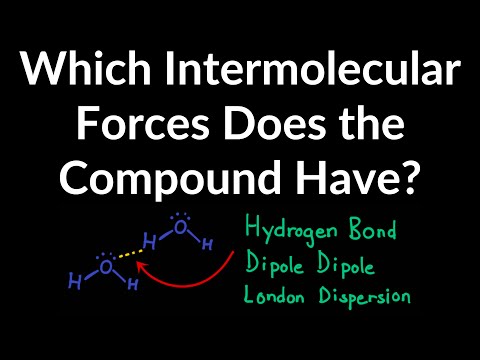
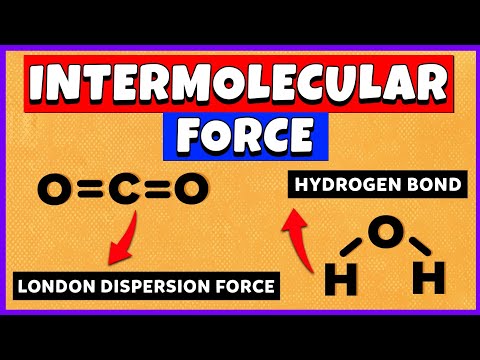
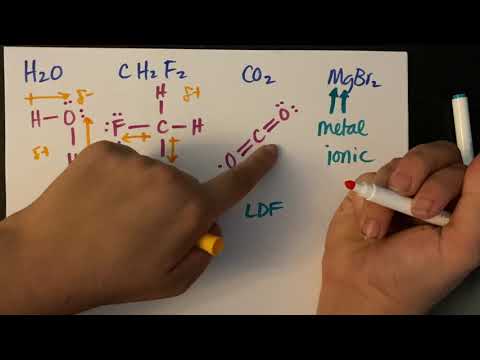
関連ワード:
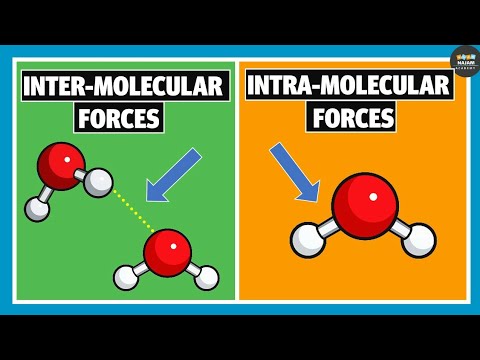
is hydrogen bonding the strongest intermolecular force why is hydrogen bond the strongest intermolecular force is hydrogen bonding a strong intermolecular force are hydrogen bonds stronger than intermolecular forces why is hydrogen bonding such a strong intermolecular force which is the second strongest intermolecular force after hydrogen bonding is dipole stronger than hydrogen bonding is hydrogen bonding an intermolecular force which has stronger hydrogen bonding