関連ワード:
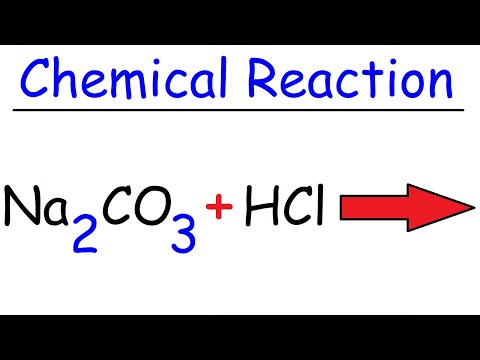
is na2co3 acidic or basic is na2co3 acidic or basic salt na2co3 is acidic or basic in nature is na2co3 acidic basic or neutral in water is na2co3 acid or base is na2co3 solution acidic or basic na2co3 is acidic basic or neutral salt is na2co3 acid base or neutral is na2co3 acid base or salt is na2co3 an acid or base in water