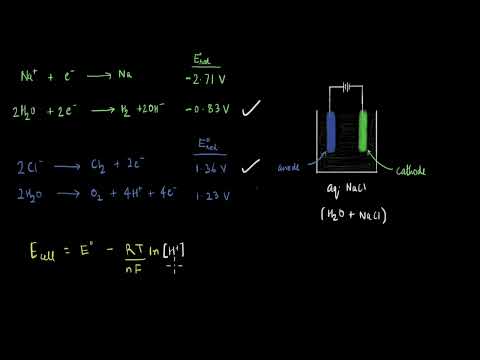
関連ワード:
is nacl an aqueous solution is nacl soluble in an aqueous solution is sodium chloride in water an aqueous solution is nacl alkaline in aqueous solution an aqueous solution of nacl is marked 10 an aqueous nacl solution is made using an aqueous solution of nacl is called an aqueous solution of nacl is electrolysed when an aqueous nacl solution is electrolyzed 20 g of nacl is added to an aqueous solution