関連ワード:
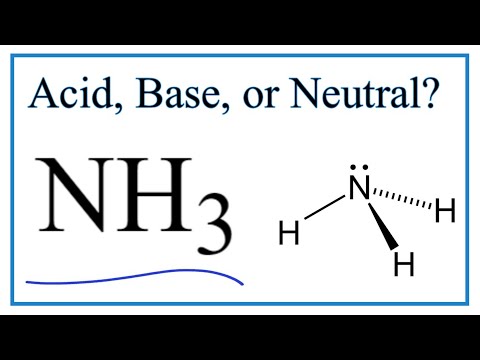
is nh3 a weak base is nh3 a weak base or acid is nh3 a weak base in aqueous solution nh3 is a weak base kb 1.8x10 5 nh3 is a weak base and so the salt nh4cl nh3 is a weak base that reacts with water nh3 is a weak base true or false nh3 is a weak base and so the salt is nh3 a strong base is ammonia a weak base