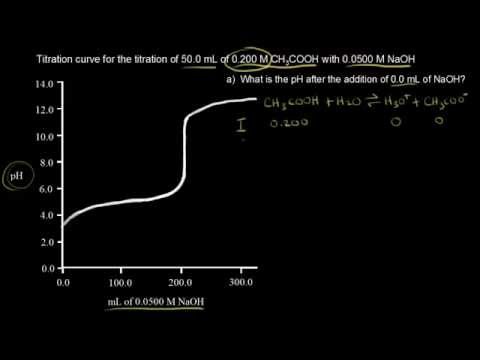
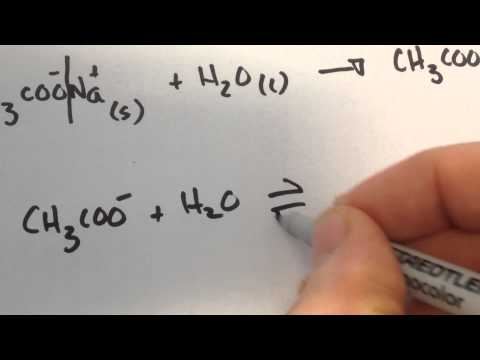関連ワード:
is sodium acetate a strong base is sodium acetate a good base is sodium acetate a strong conjugate base is sodium acetate an acid or a base is sodium acetate a strong or weak base is sodium acetate a strong acid or base is sodium acetate trihydrate an acid or base sodium acetate is a salt made up of strong base and sodium acetate is a salt made up of strong base and dash is sodium acetate a weak base