関連ワード:
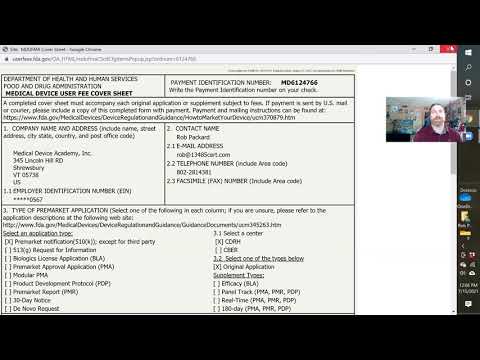
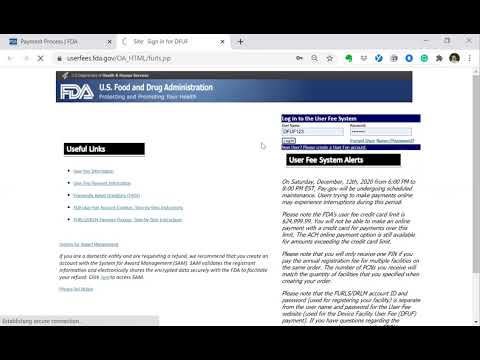
medical device registration fee medical device application fee form fda medical device registration fee mhra medical device registration fees malaysia medical device registration fee cdsco medical device registration fees sfda medical device registration fees hsa medical device registration fee medical device licence application fee form what is medical device registration