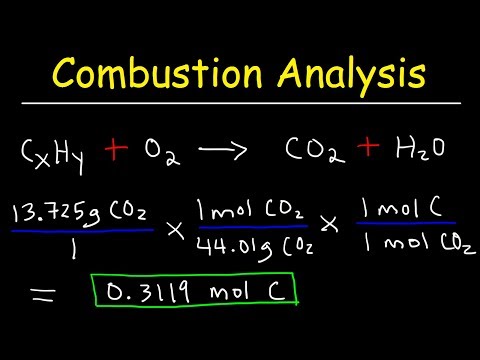
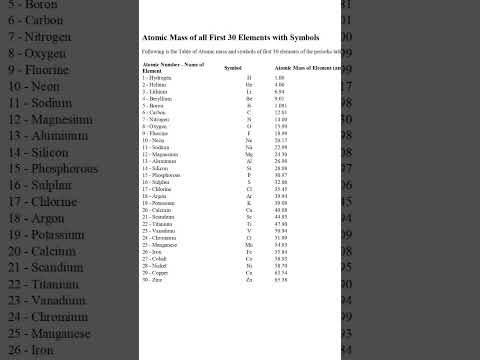
関連ワード:
molecular weight of carbon hydrogen and oxygen molecular weight of carbon hydrogen oxygen and nitrogen molecular formula of carbon hydrogen and oxygen molecular mass of carbon hydrogen and oxygen molar mass of carbon hydrogen and oxygen molecular mass of carbon 6 hydrogen 12 and oxygen 6 what is molecular weight of carbon