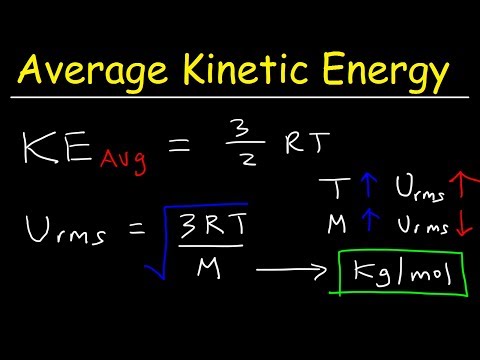
関連ワード:
root mean square velocity of a gas at temperature t root mean square velocity of a gas is tripled when the temperature is the root mean square speed of gas molecules at temperature t is directly proportional to the root mean square velocity of a diatomic gas at room temperature the root mean square velocity of a diatomic gas at room temperature is 1930 the root mean square velocity of a gas is doubled when the temperature is the root mean square speed of a gas at a particular temperature the root mean square speed of the molecules of a gas at absolute temperature t is proportional to at absolute zero temperature the root mean square velocity of a gas is the root mean square velocity of a gas molecule of mass at a given temperature is proportional to