関連ワード:
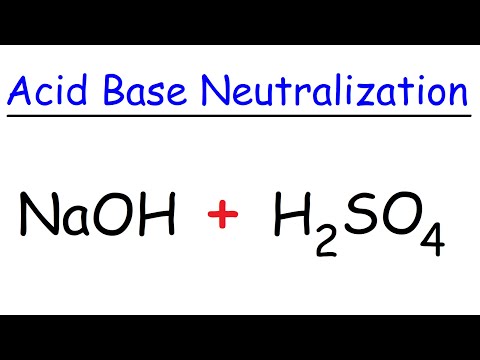
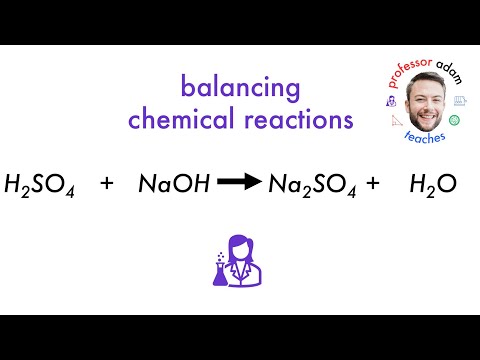
what happens when h2so4 reacts with naoh what happens when sulphuric acid reacts with sodium hydroxide what happens when sulfuric acid reacts with sodium hydroxide what happens when sulphuric acid reacts with caustic soda what happens when dilute sulphuric acid reacts with sodium hydroxide what does h2so4 and naoh make