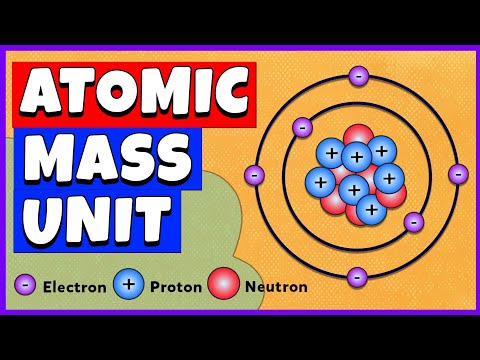
関連ワード:
what is 1/12 the mass of a carbon-12 atom what is 1/12 the mass of a carbon-12 atom in grams what is 1 12 the mass of a carbon 12 atom in grams what is 1 12 the mass of a carbon 12 atom in kg what is 1 12 the mass of a carbon 12 atom in amu what is exactly 1 12 the mass of a carbon 12 atom what is equal to 1 12 the mass of a carbon 12 atom what is defined as 1 12 the mass of a carbon 12 atom what is the unit equal to 1 12 the mass of a carbon 12 atom what is 1 2 the mass of a carbon 12 atom