関連ワード:
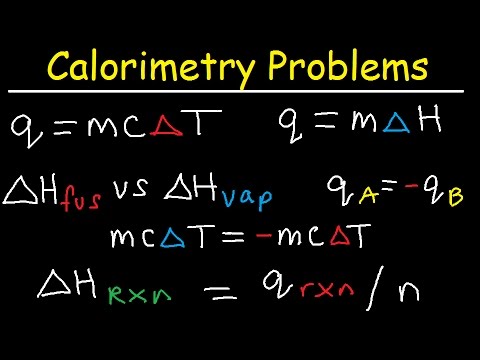
what is delta t in chemistry what is delta t in chemistry formula what is delta f in chemistry what is t delta s in chemistry what is q mc delta t in chemistry what is delta h equal to in chemistry what does delta t mean in chemistry what does delta t represent in chemistry what is delta q in chemistry