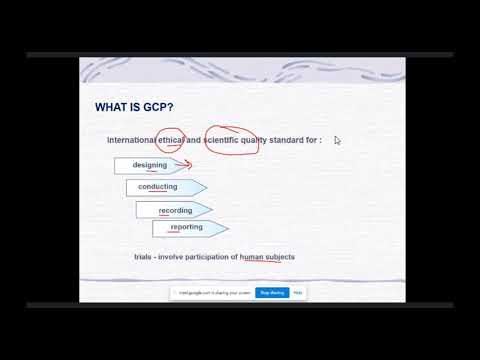
関連ワード:
what is gcp in research what is good clinical practice in research what is gcp in clinical research what is gcp training in research what is gcp certification in research what is ich gcp in clinical research what is gcp research modules what does gcp stand for in research gcp meaning in research why is gcp important to clinical research