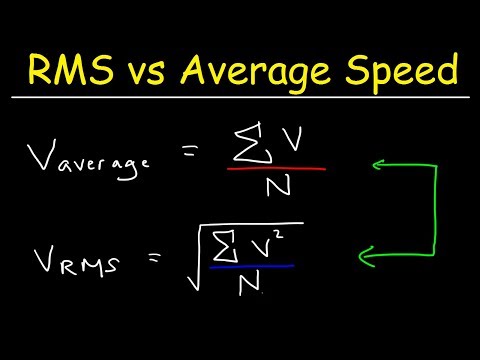
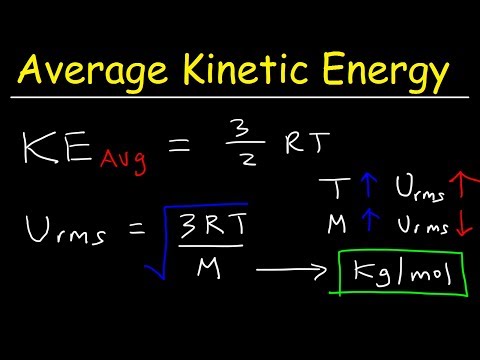
関連ワード:
what is root mean square speed of molecules what is root mean square speed of gas molecules what is root mean square speed of hydrogen molecule at 300k what is the root mean square speed of n2 molecules at stp what is the root mean square speed of oxygen molecules at 27 c what is the root mean square speed of hydrogen molecules h2 at this temperature what is the root mean square speed of helium molecules at 300 k what is the root mean square speed of oxygen molecules at this temperature what is the root mean square speed of oxygen molecules o2 at this temperature the root mean square speed of the molecules of an ideal gas is