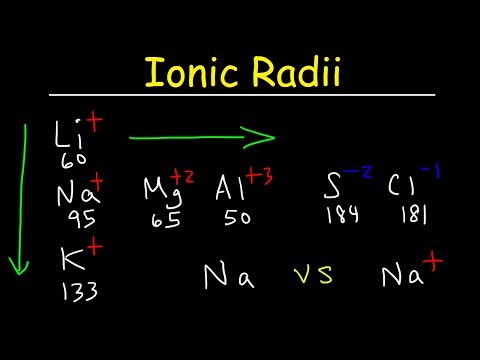
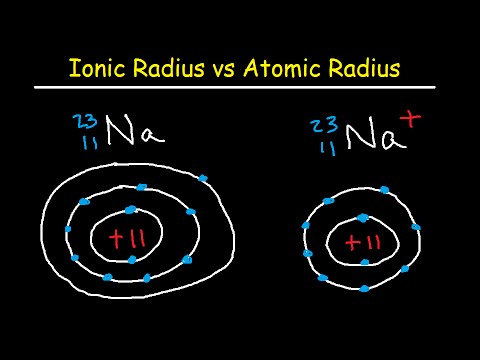
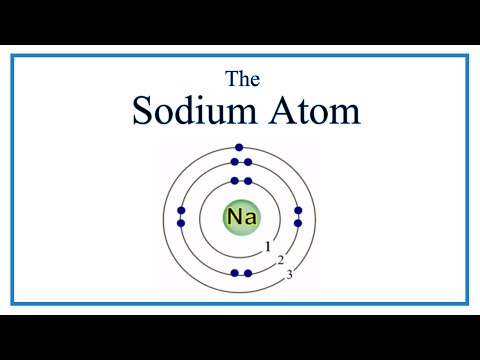
関連ワード:
what is the atomic radius of sodium what is the atomic radius of sodium in pm what is the atomic radius of sodium in picometers what is the atomic radius of sodium ion what is the atomic radius of sodium na what is the atomic radius of sodium ion in pm what is the atomic radii of sodium what is the atomic radii of sodium in pm what is the atomic size of sodium what is the ionic radius of sodium