関連ワード:
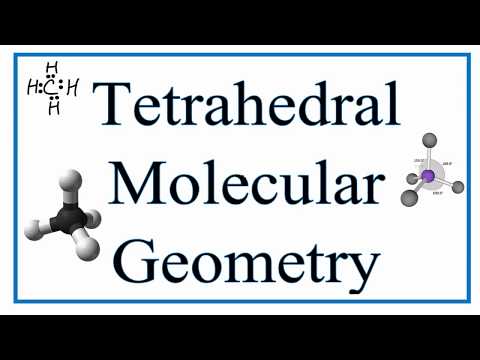
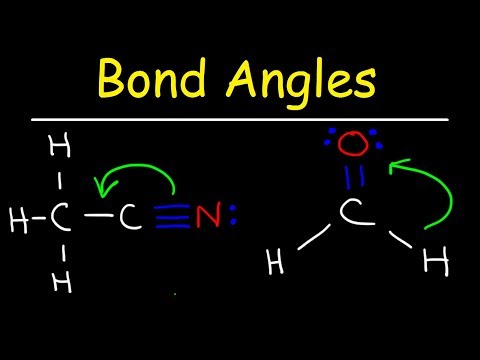
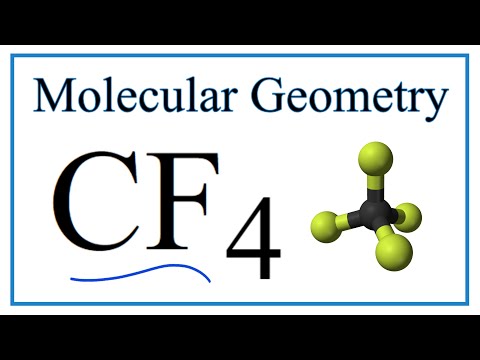
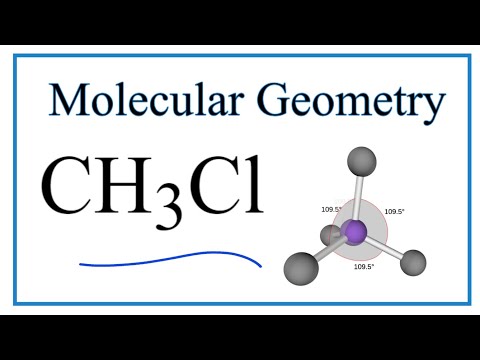
what is the bond angle of tetrahedral molecular geometry what is the bond angle of a tetrahedral shape molecule what is the bond angle of a molecule with a tetrahedral electron geometry what is the ideal bond angle in degrees of an atom with tetrahedral molecular geometry what is the bond angle of tetrahedral bond angles of molecular geometry