関連ワード:
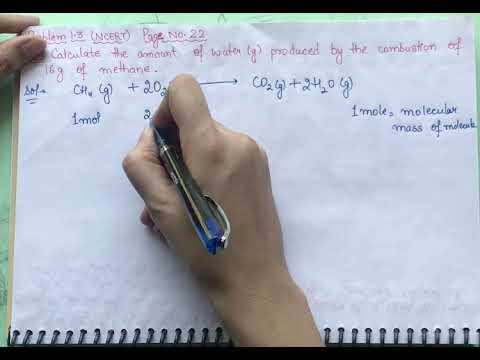
what is the mass of methane in grams what is the mass in grams of one mole of methane molecules what is the mass in grams of 1.75 moles of methane what is the mass in grams of one molecule of methane what is the mass in grams of 560 ml of methane at stp what is the mass in grams of one mole of methane what is the mass in grams of 1.75 moles of methane (ch4) what is the mass of methane what is the mass of one molecule of methane what is the mass of ch4