関連ワード:
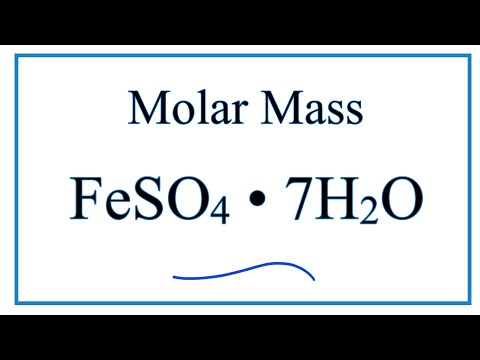
what is the molar mass of iron ii ammonium sulfate hexahydrate what is the molar mass of iron ii ammonium sulfate what is the molar mass of ammonium iron ii sulphate what is the relative formula mass of hydrated ammonium iron ii sulfate molar mass of hydrated iron ii ammonium sulfate molecular mass of hydrated iron ii ammonium sulfate