関連ワード:
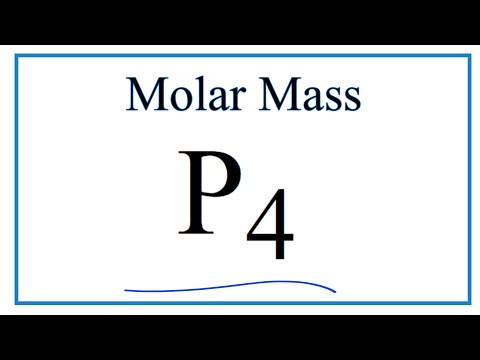
what is the molar mass of p4 what is the molar mass of p4o10 what is the molar mass of p4s3 what is the molar mass of p4o6 what is the molar mass of p4010 what is the molar mass of p4o10 to 5 significant figures what is the molecular mass of p4 what is the molecular mass of p4o10 p4s3 molecular mass what is the molecular mass of p4s10 in amu