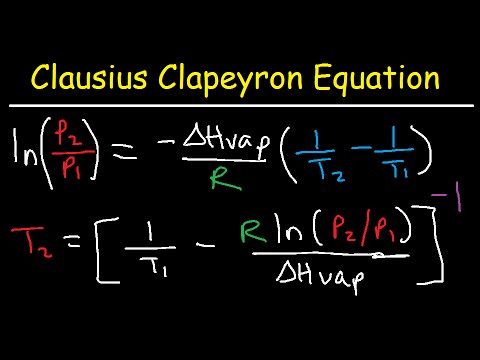
関連ワード:
which has a higher enthalpy of vaporization which has a higher heat of vaporization water or ethanol which has a higher heat of vaporization alcohol or water which has the higher enthalpy of vaporization cs2 or so3 which has the higher enthalpy of vaporization ch2cl2 or cf4 which substance has a higher heat of vaporization which should have a higher enthalpy of vaporization cf4 or cbr4 which would have a higher enthalpy of vaporization methanol or ethane which has greater enthalpy of vaporization which of the following has a greater enthalpy of vaporization