関連ワード:
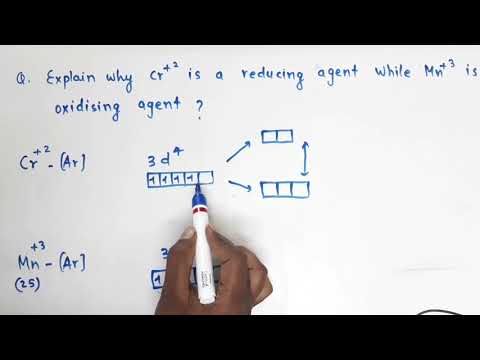
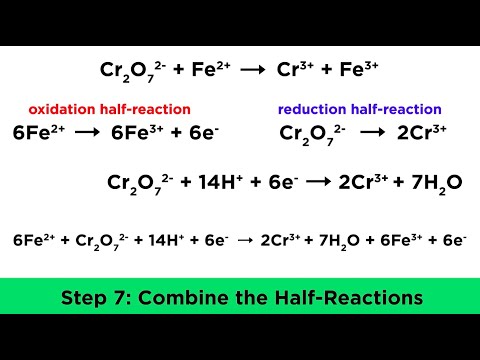
which is good reducing agent cr2+ or fe2+ which is strong reducing agent cr2+ or fe2+ which is strongest reducing agent cr2+ or fe2+ which is strong reducing agent cr2 or fe2 plus and why which is strong reducing agent cr2+ or fe2+ give reason which is strongest reducing agent cr2 or fe2 plus which is strong reducing agent cr2 positive or fe2 positive and why which is the strongest reducing agent cr2 positive or fe2 positive and why which is strong reducing agent in aqueous medium cr2+ or fe2+ and why which is stronger reducing agent cr2+ or fe2+ and why