関連ワード:
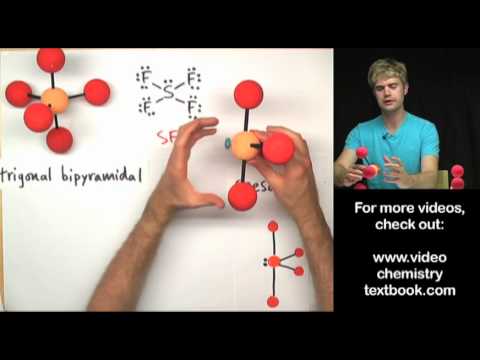
which of the following molecule is trigonal bipyramidal which of the following molecule is trigonal bipyramidal ch4 bf3 pcl5 sf6 which of the following molecule has trigonal bipyramidal geometry which of the following molecules have trigonal bipyramidal shape which of the following molecules has a trigonal bipyramidal electron geometry which of the molecule is trigonal bipyramidal according to vsepr theory which one of the following molecules is trigonal bipyramidal