関連ワード:
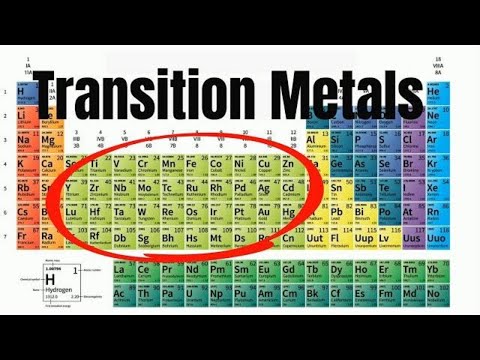
why are transition elements less reactive why are transition metals less reactive why are transition metals less reactive than group 1 why are transition metals less reactive than group 1 and 2 why are transition metals less reactive than s block elements why are transition metals less reactive than alkali metals why are bigger elements like transition metals generally less reactive why transition elements are less reactive than alkali metals are transition elements reactive are transition metal reactive