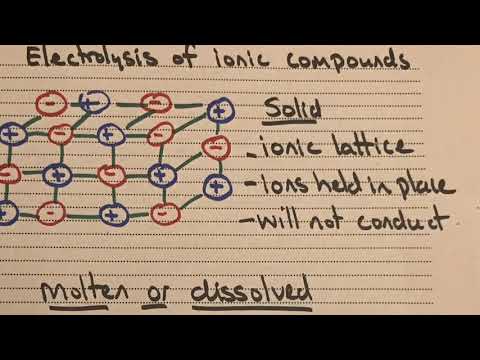
関連ワード:
why can ionic compounds not conduct electricity when solid why do ionic compounds not conduct electricity when solid gcse why do ionic compounds not conduct electricity in solid state why do ionic compounds not conduct electricity in solid form why do ionic compounds not conduct electricity in solid state class 10 why do ionic compounds not conduct electricity in the solid phase why do ionic bonds not conduct electricity in solid state why can ionic compounds conduct electricity when molten but not solid why can't solid ionic compounds not conduct electricity why do ionic compounds conduct electricity when liquid but not solid