関連ワード:
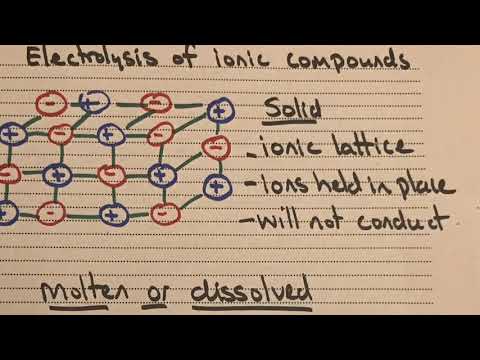
why can't ionic compounds conduct electricity when solid why can't ionic compounds conduct electricity when they are solid why can t ionic compounds conduct electricity in solid state why can't giant ionic compounds conduct electricity when in solid form why can ionic compounds not conduct electricity when solid why don t ionic compounds conduct electricity when solid why are ionic compounds unable to conduct electricity when solid why can ionic compounds conduct electricity when molten but not solid why can t ionic compounds in solid state conduct electricity well why ionic compounds cannot conduct electricity when solid