関連ワード:
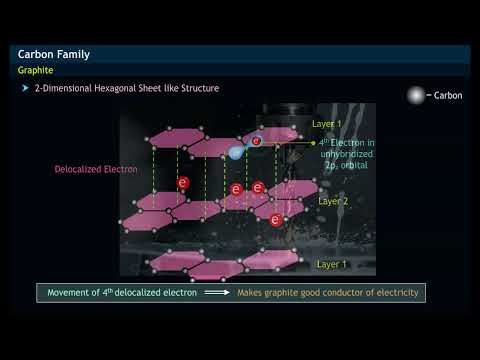
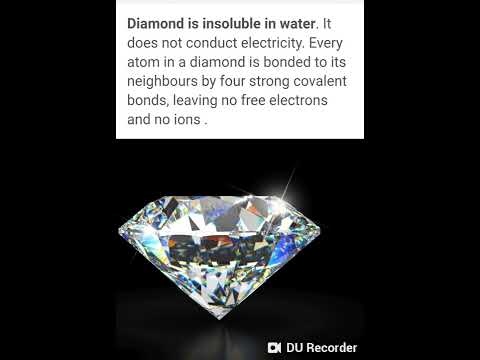
why does graphite conduct electricity but diamond cannot why can graphite conduct electricity but diamond cannot why does graphite conduct electricity but not diamond class 10 why does graphite conduct electricity while diamond does not why graphite is a good conductor of electricity but diamond not why graphite is a good conductor of electricity while diamond not why does graphite conduct electricity but not diamond