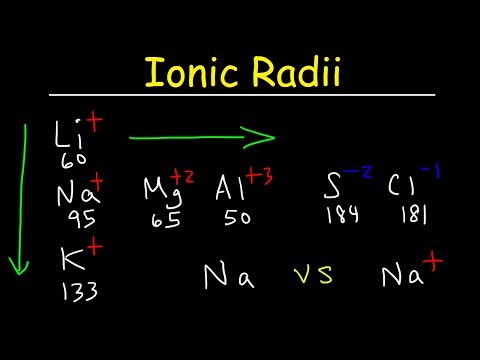
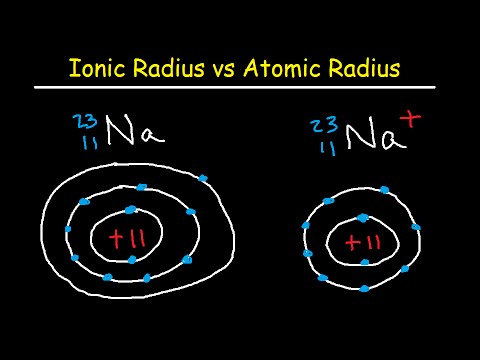
関連ワード:
why does lithium have a larger atomic radius than fluorine why does lithium have a larger atomic radius than beryllium why does lithium have a larger atomic radius than neon why does lithium have a larger atomic radius than nitrogen why does lithium have a larger atomic radius why does lithium have a larger atomic radius than oxygen why does li have a larger atomic radius than f why does li have a larger atomic radius than be why does lithium have a higher atomic radius why does lithium li have a larger atomic radius than fluorine f )