関連ワード:
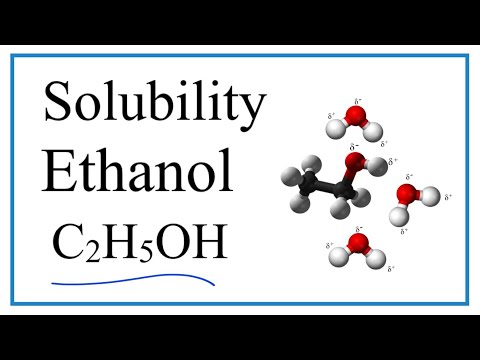
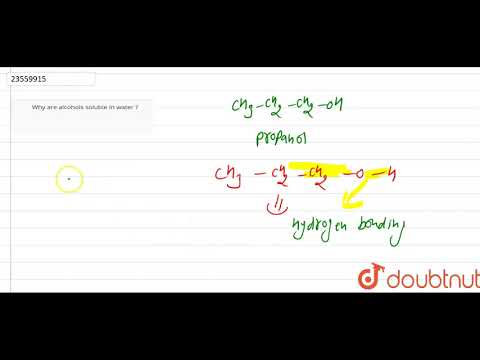
why ether is less soluble in water why ether is not soluble in water why ethers are less soluble in water dimethyl ether is less soluble in water than ethanol why ether does not dissolve in water why is diethyl ether not soluble in water why is dimethyl ether not soluble in water why alcohol is soluble in water but ether is not why ether is insoluble in water are ether soluble in water