関連ワード:
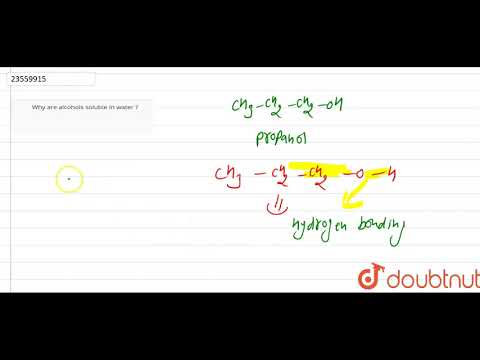
why is methanol miscible in water why is methanol soluble in water why is methanol soluble in water and gasoline why is methyl alcohol soluble in water why is methanol more soluble in water than ethanol why is methanol infinitely soluble in water why is methanol highly soluble in water why is methanol more soluble in water than butanol why is methanol more soluble in water than methane why is methanol very soluble in. water