関連ワード:
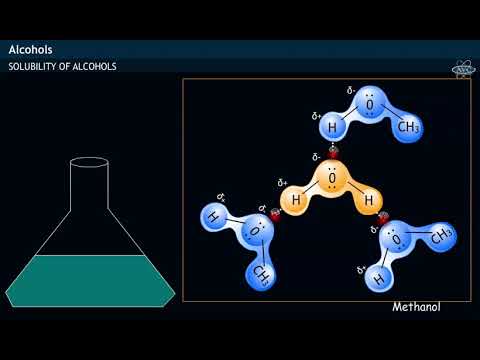
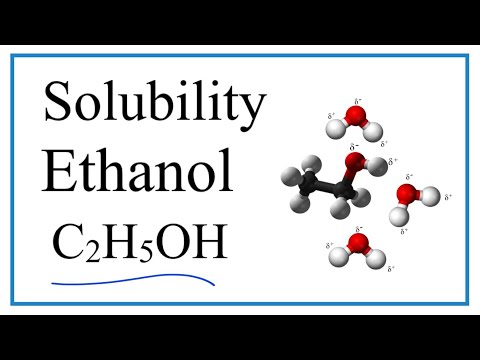
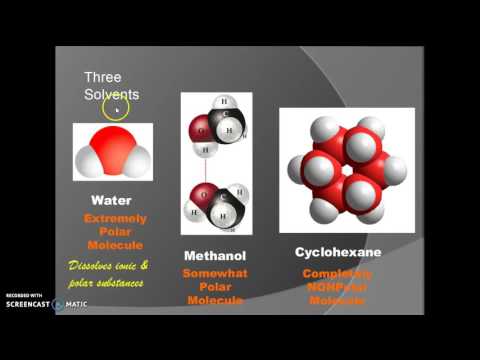
does methanol soluble in water can methanol dissolve in water and conduct electricity methanol soluble in water methanol solubility in water g l methanol solubility in water at different temperatures methanol solubility in water value methanol solubility in water mg ml methanol solubility in water g/ml does methanol dissolve in water