関連ワード:
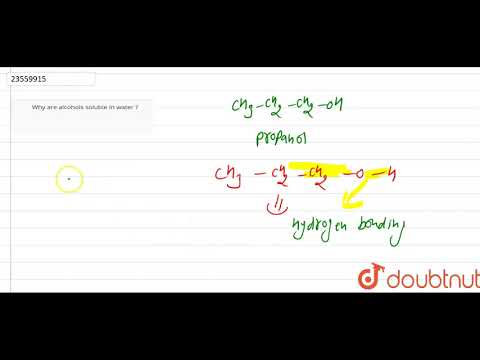
is methanol miscible in water is methanol soluble in water is methyl alcohol miscible in water is methanol soluble in water and hexane is methanol miscible in water in all proportions is methanol completely miscible in water methanol is soluble in water due to methanol is soluble in water or not methanol is soluble in water true or false does methanol soluble in water