関連ワード:
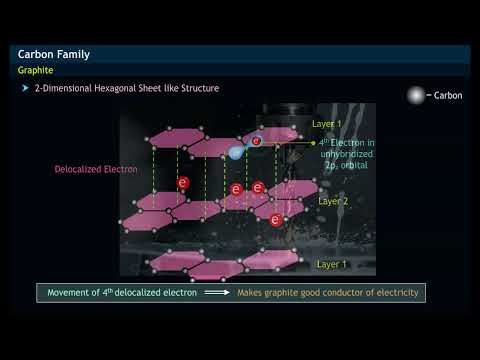
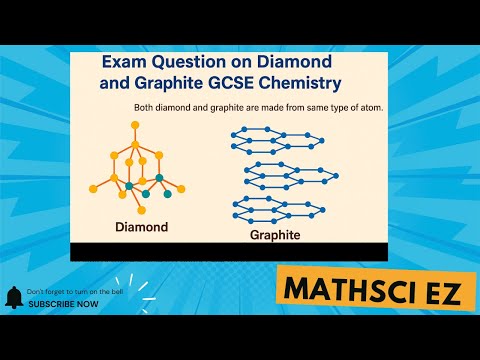
why does graphite conduct electricity and diamond doesn't why does graphite conduct electricity but diamond doesn t why does graphite conduct electricity but diamond doesn't gcse why does graphite conduct electricity but diamond cannot why can graphite conduct electricity but diamond cannot why does graphite conduct electricity but not diamond